Nuvaxovid
Sverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Contact your healthcare professional if you have any questions about the product.
Novavax S Covid 19 Vaccine Nuvaxovid Gets Conditional Approval In Switzerland Seeking Alpha
The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency EMA earlier.
. Nuvaxovid will be given to you as two separate 05 mL injections. The MHRA can confirm that Nuvaxovid does not contain any components of animal origin. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre.
The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. Novavax Nuvaxovid COVID-19 vaccine. Nu stoppar Folkhälsomyndigheten användningen bland personer.
This protein mediates the binding of the virus to the cell surface and is thus responsible for the infection of. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Nuvaxovid is administered intramuscularly as a course of 2 doses of 05 mL each.
People who had 2 doses of Novavax were about 80-90 less. The addition of the saponin-based. Publicerad idag 0702.
Approved by Health Canada. Three large clinical trials showed that Novavax is effective in preventing COVID-19 in people aged 12 years and older. Your doctor pharmacist or nurse will inject the vaccine into a muscle usually in your upper arm.
Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. It is recommended that. The use of this vaccine should be in accordance with.
Novavaxin Nuvaxovid-koronarokote antaa suojaa SARS-CoV-2 viruksen aiheuttamaa infektiota ja COVID-19 -tautia vastaan. 2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. Vial and carton labels with English-only labelling.
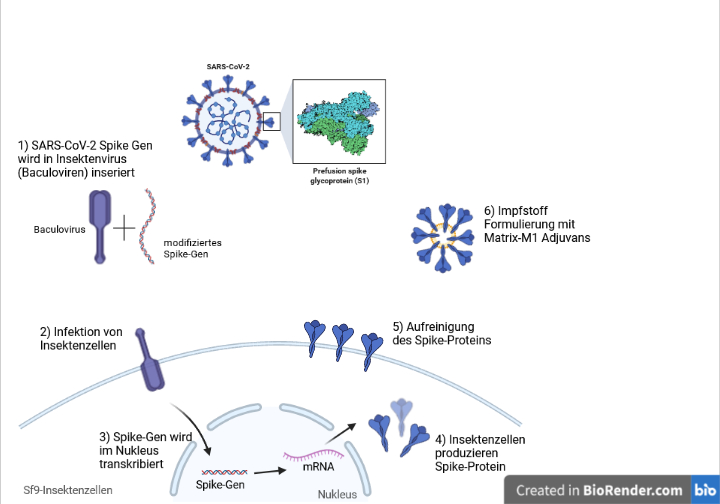
A full list of ingredients for the qualitative and quantitative composition of the. Nuvaxovid contains a version of a protein found on the surface of SARS-CoV-2 the spike protein of the virus that causes COVID-19 which has been produced in the laboratory. 1122022 64005 AM.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. The European Commission will now fast. The protein-based covid-19 vaccine Nuvaxovid should not be given to people who are 30 and younger announces the Public Health Agency.
Rokote ehkäisee erittäin tehokkaasti etenkin vakavaa. The subunit that is used here as vaccine is the spike protein S of SARS-CoV-2. Nuvaxovid has manufacturing sites in the Czech Republic Australia Canada Japan and South Koreas SK.
Beslutet är temporärt och gäller från. Nuvaxovid is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in individuals 12 years of age and older. Unlike mRNA vaccines such as Pfizer and Moderna the Novavax vaccine uses a longer-standing protein-based technology.
It is recommended to administer the second dose 3 weeks after the first dose see section 51. 19 2022 Novavax signed agreements with. Nuvaxovid contains a version of a protein found on the.
Nuvaxovid is given as two injections usually into the muscle of the upper arm 3 weeks apart. Company Novavax should not be given to individuals younger than 30 the Public Health Agency of. Det eftersom att data.
Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Age 18 and older. The flu vaccine and the hepatitis B vaccine which.
What Is The Novavax Vaccine And Why Does The World Need Another Type Of Covid 19 Vaccine Gavi The Vaccine Alliance
Nuvaxovid Gets Expanded Provisional Approval In Nz As Covid 19 Booster For Adults
Nuvaxovid The New Subunit Sars Cov 2 Vaccine Mci Innsbruck

Middlesex London Health Unit Are You Interested In The Novavax Nuvaxovid Covid 19 Vaccine Mlhu Is Receiving A Limited Supply Of The Vaccine This Spring Call 1 226 289 3560 To Be Added To The Waitlist
Faq What You Need To Know About Novavax S Non Mrna Covid 19 Vaccine Nuvaxovid Cna

Cdc Endorses Novavax Covid Shot For Adults Fortune
Who Lists 10th Covid 19 Vaccine For Emergency Use Nuvaxovid Strategic Partnership For Health Security And Emergency Preparedness Sph Portal

Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022
Fda Committee Oks Novavax S Late To The Game Covid 19 Vaccine Cbs News

Nuvaxovid Dispersion For Injection Covid 19 Vaccine Recombinant Adjuvanted Patient Information Leaflet Pil Emc
News Conditional Marketing Authorisation Application Submitted For Novavax S Covid 19 Vaccine Nuvaxovid Paul Ehrlich Institut
After A Decent First Quarter Novavax S Covid Shot Is Struggling

Novavax Covid Vaccine Nuvaxovid Medcity News

Novavax S Covid 19 Vaccine Approved For Canadians 18 And Older Cbc News
Fda Authorizes Novavax Covid 19 Vaccine For Emergency Use In Us Abc News

The Fda S Decision On The Novavax Covid 19 Vaccine Could Come In Weeks Marketwatch

Covid Vaccine Novavax Requests Who To Expand Emergency Use Listing Of Nuvaxovid For Adolescents Aged 12

Nuvaxovid Covovax Novavax Vaccine Covid 19 Info Vaccines

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care